Schizophrenia Research at the National Institute of Mental Health
An overview that summarizes research into the causes, diagnosis, prevention, and treatment of schizophrenia 2005
Schizophrenia Research at the National Institute of Mental Health
Since the Institute's inception 50 years ago, much has been learned about mental disorders and their effects on the brain. Revolutionary scientific advances in neuroscience, molecular biology, genetics, and brain imaging have provided some of the greatest insights into the complex organ that is the seat of thought, memory, and emotion. Thanks to these new tools, the scientific evidence that mental illnesses are brain disorders now exists.
What Causes Schizophrenia?
The causes of schizophrenia are still unknown, but they are bound to be complex, since the disease affects perception, memory, attention, cognition, and emotion — some of the most highly evolved functions in humans. Recent research suggests that a number of factors likely interact with each other to produce schizophrenia. These factors include heredity, events during development that affect the brain (such as viral infections during pregnancy), environmental stressors (such as exposure to pollutants or toxins), psychological stress, and others. There appear to be a variety of recipes that result in the development of the disease. Indeed, what we call "schizophrenia" may ultimately be traced to different underlying processes and the diagnostic terms may someday change.
Some people may develop schizophrenia even if they grow up and live in the best possible environment. Such individuals may have inherited a large number of risk genes or sustained such significant early brain damage that nothing can compensate for it. Others may carry predisposing genes but not develop the disease unless they experience certain environmental stressors. The likelihood that heredity alone is not enough is borne out by the fact that sometimes one identical twin will develop schizophrenia and the other will not, even though they share the same genes. Researchers are trying to identify the risk factors and to discover how they work together to produce schizophrenia.
The Neurodevelopmental Hypothesis
Most people who develop schizophrenia don't start showing symptoms until they reach late adolescence or young adulthood. This poses one of schizophrenia's most perplexing mysteries: How might effects of early damage remain dormant for the first two decades of life and then strike just as people enter their most productive years? Such latent damage in the developing brain might not come "online" until key circuitry fully matures, some researchers propose. They point to evidence that faulty wiring is laid down in schizophrenia during fetal development. Unable to cope with the stresses of young adulthood, the flawed circuitry breaks down, resulting in psychotic symptoms, according to this neurodevelopmental theory. Alternatively, researchers have proposed that the neurodevelopmental processes that occur during adolescence are the ones that are altered[1]
Studies in rare teens who developed schizophrenia prior to puberty are providing clues. While it's normal for teens to lose some unused neural connections as their brains mature, MRI scans reveal a runaway pruning process in teens with schizophrenia. They lose neural connections in a wave that envelopes the brain from back-to-front (link to animation), culminating in the frontal lobes, where gray matter wanes four times faster than in their healthy peers. The relatively late maturation of this schizophrenia-linked area coincides with the usual age-of-onset of schizophrenia. The researchers suggest that brains of adult-onset patients may have undergone a similar process prior to developing symptoms.[2]
The faulty wiring in schizophrenia might be traceable, in part, to versions of genes that create too many or too few of the proteins that help neurons develop and migrate properly in the developing brain. Viruses or parasites the mother catches while she is pregnant might also affect these processes, as might toxins she is exposed to through breathing, eating, drinking, and smoking. Identifying which elements in the environment are associated with schizophrenia may help prevent the disease.
Environmental Factors
Environmental factors associated with schizophrenia include toxins, pollution, and viral exposure; malnutrition; emigrating to another country; being born in winter; being born in a city; and childhood brain injury. Since most people who experience such stressors do not develop schizophrenia, vulnerability seems to hinge on an interaction between environmental factors and underlying conditions in the brain.
For example, the first study based on influenza antibodies in archived blood samples recently found that if a pregnant mother caught the flu during the first trimester it increased schizophrenia risk in her offspring 7-fold. This suggests that giving flu vaccinations to women of reproductive age might prevent some cases of schizophrenia. However, scientists have not ruled out the possibility that damage to the developing brain might be caused by a runaway antibody response to the influenza virus rather than by the infection itself. In that case, such women might be advised to avoid flu shots, which trigger an antibody response, albeit much weaker than the response triggered by the flu itself. More studies are needed determine if there are any such possible effects.[3]
The same NIMH-funded research team also recently reported the first antibody evidence that toxoplasmosis, a parasitic infection, doubles or triples a mother's chances of giving birth to a child who will develop schizophrenia. Toxoplasmosis can be prevented by such common sense measures as cooking meat thoroughly, washing vegetables and fruit, and washing one's hands after gardening or changing cat litter. Again, the schizophrenia risk may not stem from the infection itself, but rather from the body's reaction to it, such as the inflammatory response.[4]
A clue emerged recently when the researchers found that levels of a key immune system chemical secreted in response to infection and inflammation were abnormally high in pregnant mothers whose children later developed schizophrenia spectrum disorders. Blood levels of interleukin-8 were twice as high during the second trimester in these mothers compared to mothers whose children did not develop the illness. No such differences turned up in levels of three other such cytokines examined. The boost in interleukin-8 could reflect a maternal infection or some other inflammatory process that increases schizophrenia risk. Some of interleukin-8's properties suggest that it could contribute to the type of abnormal brain development suspected of occurring in schizophrenia.[5]
Genetic Factors
The recent mapping of the human genome has provided information and tools with the potential for changing the face of psychiatry. Clues are already emerging about how an individual will respond to a medication differentially based on the version of a gene he or she has inherited.[6] Someday soon, doctors will prescribe drugs based on the patient's unique genetic profile; drug companies are already gearing up to design medications targeted at particular mixes of genes.
The revolution in genetics is of particular importance for schizophrenia. Family, twin, and adoption studies have shown that the tendency to develop schizophrenia is at least 60 percent inherited. If one's parent or sibling has schizophrenia, one has a 10 percent chance of developing the disease (compared to 1 percent in the general population). If the person with schizophrenia is an aunt, uncle, cousin, or grandparent one is still at greater risk; and if an identical twin has schizophrenia the probability rises to between 40 and 65 percent.[7],[8]
Yet, no one has determined precisely how this occurs. Many locations on almost every one of our 23 pairs of chromosomes have been associated with schizophrenia. Seven of these genes (on chromosomes 1, 6, 8, 13, 15, and 22) have particularly strong links to the disease, but, so far, none have been shown to account for more than a small fraction of the vulnerability.[9]
Some genes that have recently been associated with schizophrenia code for enzymes and proteins that help brain cells communicate with each other. Some of these enzymes and proteins are involved in neurotransmitter systems that have long been implicated in schizophrenia, such as dopamine, glutamate and GABA. Other genes code for proteins involved in brain development, while others code for proteins of yet undetermined funciton.
"Candidate" Genes
Genes that produce substances that interact with
neurotransmitters to either block them or reduce their
availability are also being studied. The following table
lists some of the candidate genes thought to increase
risk for schizophrenia and explains what they do.
Schizophrenia Candidate Genes
| GENE | CHROMOSOME | FUNCTION |
|---|---|---|
| COMT (catechol-O-methyl transferase) | 22q11 | Enzyme that breaks down dopamine. People who have the val version have low dopamine levels in their prefrontal cortex and do not perform as well on cognitive tasks as people with the met version (see below). |
| Dysbindin (DTNBP1) | 6p22 | Increases the amount of glutamate in the brain, which interferes with the action of the neurotransmitter NMDA (N-methyl-D-aspartame). NMDA is necessary for memory, learning, and being able to adapt to changing circumstances in the environment. |
| Neuregulin 1 (NRG1) | 8p12-21 | Regulates many brain cell functions. It affects how well brain wiring develops in the womb, how well neurotransmitters work, and how well the brain can grasp and adapt to new situations. |
| GRM3 | 7q21-22 | Alters glutamate levels in the prefrontal cortex and the hippocampus, and thereby affects higher cognitive functions. |
| DISC1 (disrupted-in-schizophrenia 1) | 1q42 | Strongly linked to psychopathology, including schizophrenia, depression, and mania, but its biological function has not yet been determined. It is found most often in the brain's limbic system, which controls emotions. |
| G72 and DAAO | 12q32-34 and 12q24 | Activates DAAO (D-amino acid oxidase), which promotes the action of NMDA (see Dysbindin). Like GRM3, COMT and DISC1, G72 may promote schizophrenia by disrupting the functioning of the prefrontal cortex and hippocampus. |
| CHRNA7 (alpha-7 nicotinic receptor gene) | 15q13-14 | Regulates how sensitive the system is to nicotine, which slows down attention and sensory processing. CHRNA7 also helps promote good dopamine and glutamate signaling between neurons. This gene is seen less often in the cells of people with schizophrenia, especially in brain regions often implicated in this disorder, such as the hippocampus, thalamus, frontal cortex and the cingulate cortex. |
| PRODH2 | 22q11 | Helps regulate the levels of the enzyme proline. When proline levels are too high, glutamate levels in the brain drop too low. |
| PPP3CC | 8p21 | A subunit of the calcineurin gene, PPP3CC helps dopamine and glutamate transmit messages from neuron to neuron. |
| RGS4 (regulator of G-protein signaling 4) | 1q21-22 | Helps neurons mature and is part of the way neurotransmitters work inside neurons. |
How Genes Work to Increase Risk
Researchers are just beginning to understand how some of these candidate genes might increase risk. Genes don't code directly for delusions, hallucinations, flat affect and other symptoms. Genes code for proteins. So how might variations in proteins ultimately result in schizophrenia? Researchers are trying to discover the functional consequences of tiny glitches in genes, called polymorphisms, where the chemical sequence varies slightly in the population, resulting in different versions of a gene. The collective effect of even small variations in multiple genes can likely bias the brain toward schizophrenia symptoms through effects on proteins that shape the developing brain, regulate communication between brain cells, and perform other functions yet to be identified.
For example, individuals inherit two copies (one from each parent) of the gene for the enzyme catecho-O-methyltransferase (COMT), which breaks down the neurotransmitter dopamine, long suspected of going awry in schizophrenia. (Antipsychotic drugs work by blocking dopamine.) The COMT gene comes in two common versions, val and met, so a person can have two of the same version or one of each. Although the two versions differ only slightly in their molecular sequence, recent studies show that they have different effects on dopamine. Since it results in considerably stronger enzyme action, people with the more common val version are thought have less dopamine in the prefrontal cortex and perform worse on tasks involving this area. Such "executive" functions — working memory, motivation and learning in response to reward — are impaired in schizophrenia, which is thought to involve a dopamine imbalance. Studies have shown that inheriting two copies of the val version leads to a slightly higher risk for schizophrenia and a telltale pattern of midbrain dopamine / prefrontal cortex activity that is the opposite of that seen in people with the met version. Brain scans of patients performing working memory tasks have linked the val version to inefficient prefrontal activation and poorer response to antipsychotics. New evidence also suggests that the val version may interact with environmental stressors, such as teenage marijuana use, to increase risk.[10],[11]
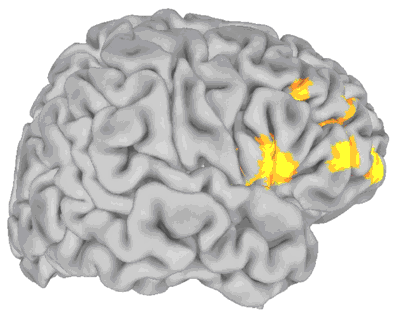
Areas in prefrontal cortex where blood flow (yellow) was linked to midbrain dopamine synthesis, in opposite directions in subjects with val and met COMT gene type. PET data is superimposed on 3-D MRI view of brain.
(See: Brain Scans Reveal How Gene May Boost Schizophrenia Risk, http://www.nimh.nih.gov/press/schizcompt.cfm)
NIMH Research Initiatives on Schizophrenia
The Genes, Cognition and Psychosis Program in NIMH's Intramural Research Program at the NIH campus in Bethesda, Maryland, uses animal models, molecular genetics tools, cell culture, postmortem studies, and brain imaging techniques to explore how genes affect the way we think and how they influence the development of psychosis.[12]
For example, the NIMH Genetic Study of Schizophrenia focuses on gathering genetic and environmental information on families with at least one member suffering from schizophrenia in order to better understand what places one family member at risk and conversely, what protects other family members. To this end, family members are asked to participate in a number of neuropsychological tests, clinical assessments and imaging studies that help scientists answer these questions.[13]
Outside of its own laboratories, NIMH funds the work of scientists at academic centers throughout the US and sometimes in other countries. For example, NIMH funds large-scale genome scans to pinpoint schizophrenia-linked genes and sponsors the NIMH Human Genetics Initiative and Genomic Resources Program, which collects data on families with a history of mental illness and distributes it to the scientific community around the world. This program has grown into a massive repository of information on gene mapping for schizophrenia and other psychiatric disorders, the interaction between genetic factors and the environment, and the brain systems and neural circuits associated with mental illness.[14]
In addition to genetic initiatives, NIMH supports many different research projects on schizophrenia, asking questions about its biological basis, studying high risk populations, attempting to identify ways to prevent it and improve its treatment.
For example, the Silvio O. Conte Centers for the Neuroscience of Mental Disorders supports six research teams dedicated to translating and integrating basic and clinical neuroscience on schizophrenia. Each of these multidisciplinary teams at the University of Pittsburgh, University of Pennsylvania, Washington University, Harvard University, Yale University, and Mt. Sinai School of Medicine is pursuing highly focused research driven by a single hypothesis related to environmental, genetic and other biological factors in brain development, structure and function related to schizophrenia.[14]
Some researchers believe that may be possible to arrest the course of schizophrenia by intervening early, before truly psychotic symptoms begin. During this “prodromal” period, adolescents destined to develop schizophrenia often experience thinking, mood and behavioral disturbances. Catching these problems early and treating with medication and other therapies might help them recover from the full-blown illness. NIMH is funding a North American Prodromal Longitudinal Study that prospectively follows teens at risk to characterize this phase, validate diagnostic criteria and improve risk prediction. A total of ten investigators are participating at eight sites - Yale University, Emory University, University of California San Diego, Harvard University, University of North Carolina, Hillside Medical Center, University of California Los Angeles, and the University of Toronto. By using a common method for assessing risk and pooling their data, the researchers are boosting the statistical power of their findings, which could eventually help inform even more aggressive preventive treatments designed to alter the fundamental course of schizophrenia.
A new multi-Institute basic neuroscience effort, the Molecular Libraries and Imaging Initiative, is a part of the NIH Roadmap for which NIMH has assumed a lead role. The goal of this effort is to provide organic chemical compounds, known as "small molecules," to scientists to use as tools to improve our understanding of biological pathways in health and disease. This is of particular interest for schizophrenia research because of the small number of molecules studied in psychiatric disorders and targeted for their treatment. The development of highly sensitive brain imaging probes will likely provide invaluable information about brain circuits involved in mental illness and those that are altered by treatment. Grants were recently awarded totaling $88.9 million to nine institutions over three years to establish a collaborative research network that will use high-tech screening methods to identify the compounds.
The explosion of data about the brain is overwhelming conventional ways of making sense of it. Like the Human Genome Project, the Human Brain Project is building shared databases in standardized digital form, integrating information from the level of the gene to the level of behavior. Under NIMH's leadership, fifteen federal organizations across four agencies fund a system of web-based databases and research tools that help brain scientists share and integrate their raw, primary research data. During the initial 10 years of this program 241 investigators have been funded for a total of approximately $100 million.
To advance research by fostering collaborations and promoting interactions among schizophrenia researchers, the NIMH has awarded a contract to develop the Schizophrenia Research Forum (SRF), a website developed by a group of science writers and web designers in collaboration with the Mental Health Research Association and the National Alliance for Research on Schizophrenia and Depression (NARSAD). The site will include new publications, a database of citations, tools for research and moderated live chats for scientists. The SRF will be independent, nonprofit, and open to all users free of charge. Although dedicated to the more than 8,000 people in volved in schizophrenia research, most features of the site will be open to the public.
Treatment Development for Cognition in Schizophrenia
The cognitive symptoms of schizophrenia — difficulties with attention, memory, and problem solving — can create significant barriers to a normal and productive life. Finding treatments for them has been hampered by a lack of scientific consensus on which cognitive impairments should be targeted for research and what tools are best for measuring them. As a result, the Food and Drug Administration (FDA), which oversees all research on human drugs and determines whether a drug is effective and safe enough to be sold, has not yet been able to designate cognition in schizophrenia as a valid treatment goal for industry-sponsored research.
To address these issues, NIMH established the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) program. MATRICS researchers look at the brains of both healthy people and people with schizophrenia to see how they function. The information they obtain on brain functioning during problem solving tasks will be used to identify potential molecular targets for new cognition-enhancing drugs. In addition, the investigators are exploring better ways to conduct clinical trials for testing compounds that might improve cognition, and are developing collaborations between government, academic, and industrial researchers to test the compounds when they are identified. MATRICS sponsored a collaboration in which academic institutions, industrial entities, and regulatory agencies worked together to develop a new standardized test to measure cognitive functioning in people with schizophrenia. This will help determine whether medications designed to improve cognition are working. Recommendations developed through MATRICS collaborations for identifying cognitive targets and improving clinical trial design were also presented to NIMH and the FDA and are currently being combined into standard consensus guidelines for testing cognition-enhancing drugs. With better guidelines regarding FDA requirements for achieving approval for new drugs, pharmaceutical companies will have greater incentives to work on this important area.
In May 2004, NIMH awarded a four-year, $9 million contract to create a network of Treatment Units for Neurocognition in Schizophrenia (TURNS). These units were established at seven research sites across the nation and focus on refining the experimental methods used to assess drug compounds, identifying promising treatments, and conducting clinical trials. Using MATRICS guidelines, TURNS represents the next step in NIMH's treatment development initiative. In the summer of 2005, the TURNS Compound Selection Committee began its second nationwide "call for nominations" seeking new therapeutic compounds to test their ability to treat cognitive deficits in schizophrenia.
References
[1] Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005 May;10(5):434-49.
[2] Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11650-5.
[3] Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004 Aug;61(8):774-80.
[4] Brown AS, Schaefer CA, Quesenberry CP Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005 Apr;162(4):767-73.
[5] Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004 May;161(5):889-95.
[6] Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003 May 13;100(10):6186-91. Epub 2003 Apr 25.
[7] Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004 Jun 19;363(9426):2063-72.
[8] Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000 Spring;97(1):12-7. Review.
[9] Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005 Aug;10(8):804.
[10] Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005 May;8(5):594-6. Epub 2005 Apr 10.
[11] Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003 Mar 15;23(6):2008-13.
[12] http://cbdb.nimh.nih.gov/
[13] http://cbdb.nimh.nih.gov/sibstudy/index.html
[14] http://www.nimh.nih.gov/dnbbs/7g-grr.cfm
[15] http://grants1.nih.gov/grants/guide/pa-files/PAR-98-058.html
- NIMH does not endorse or recommend any commercial products, processes, or services, and publications may not be used for advertising or endorsement purposes.
- NIMH does not provide specific medical advice or treatment recommendations or referrals; these materials may not be used in a manner that has the appearance of such information.
- NIMH requests that non-Federal organizations not alter publications in a way that will jeopardize the integrity and "brand" when using publications.
- Addition of Non-Federal Government logos and website links may not have the appearance of NIMH endorsement of any specific commercial products or services or medical treatments or services.
If you have questions regarding these guidelines and use of NIMH publications, please contact the NIMH Information Center at 1-866-615-6464 or at [email protected].




